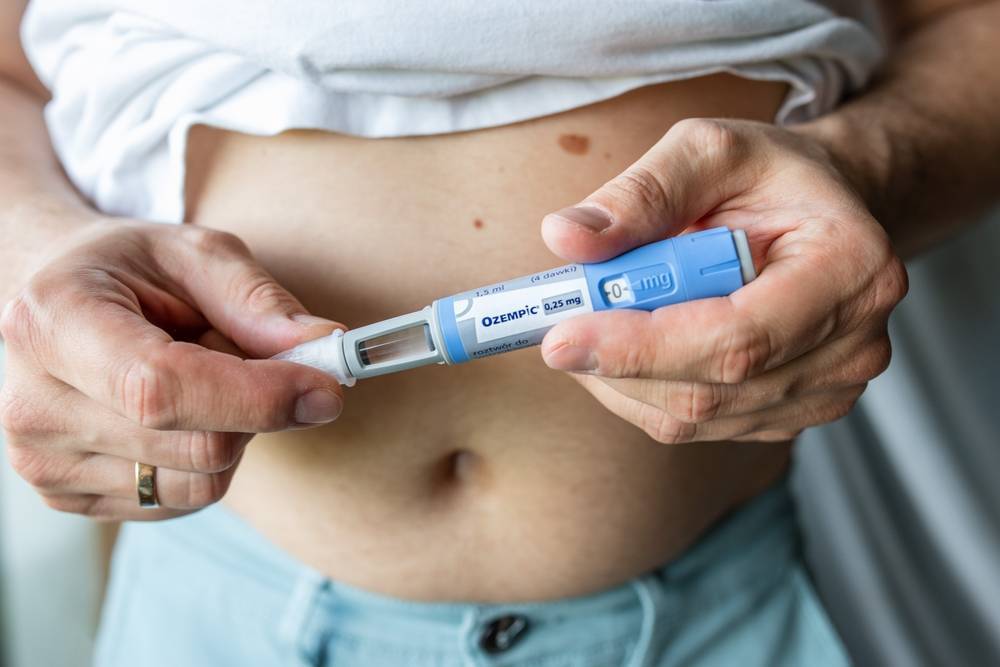
The Food and Drug Administration’s (FDA) warning about non-branded alternatives to Ozempic and Wegovy highlights the risks posed by unregulated options.
A recent warning issued by the FDA stated they had received reports of problems faced by patients after using compounded versions of the active ingredient in two widely prescribed medications: Ozempic and Wegovy.
The report warned against modified glucagon-like-peptide-1 (GLP-1) drugs, like semaglutide. Some non-branded alternatives may contain a compounded version of the active ingredient found in a drug. These versions are mixed in pharmacies and may not have approval for use in people.
Growing Prevalence of Ozempic and Wegovy
Ozempic and Wegovy are primarily prescribed to treat type 2 diabetes and obesity, and their popularity has surged due to their effectiveness in managing these conditions. However, an increasing demand for both these medications has led to their shortage, and the emergence of non-branded alternatives, which are produced by compounding pharmacies.
When drugs are in short supply, regulatory authorities permit compounding pharmacies to produce versions of those medications. Patients may find these non-branded alternatives appealing due to their lower costs and easier accessibility. However, it is important to note that these compounded versions do not have FDA approval.
FDA Concerns On Non-Branded Alternatives to Ozempic and Wegovy
The FDA’s primary concern regarding non-branded alternatives of Ozempic and Wegovy lies in the uncertainty of their composition and quality. These alternatives are not subject to the same rigorous testing and regulatory oversight as the branded medications are.
Therefore, without FDA oversight, there is no assurance that they meet quality and safety standards. Such variations could potentially compromise the effectiveness and safety of these medications. The notice states that the FDA does not approve compounded drugs and does not verify the safety or effectiveness of such drugs.
Importance of Regulatory Oversight
The FDA’s warning emphasises the importance of regulatory oversight when it comes to medications. Branded medications undergo rigorous testing and quality control measures to ensure their safety and efficacy.
Regulatory authorities like the FDA play a crucial role in safeguarding public health by evaluating and approving medications based on scientific evidence. Deviating from regulated medications poses risks to patients and may undermine trust in the healthcare system. Any variations in the formulation and quality of these alternatives may lead to inconsistent dosing, inadequate efficacy, or unexpected adverse reactions. And patients that rely on these medications to manage their conditions may face serious consequences. It is crucial to consult healthcare professionals and obtain prescribed medications from trusted sources to ensure optimal safety and efficacy.
Patient Education and Awareness
Informing patients about the potential risks of non-branded alternatives is necessary, to ensure their safety and well-being. Healthcare professionals should engage in open communication and discussions with their patients.
They should provide patients with accurate information about the benefits and risks associated with Ozempic and Wegovy, as well as the potential dangers of non-branded alternatives. Empowering patients to make informed decisions about their treatment options can help prevent the use of potentially harmful medications.
The Bottom Line:
This warning reminds both healthcare professionals and patients of the potential risks associated with non-branded alternatives of Ozempic and Wegovy. Factors such as cost and accessibility are driving the increasing prevalence of these alternatives.
However, it is essential to prioritise patient safety by adhering to prescribed medications from trusted sources. Regulatory oversight and patient education also play a crucial role in mitigating the risks associated with non-branded alternatives. By staying informed and vigilant, we can ensure that patients receive the safest and most effective treatments for type 2 diabetes and obesity.
Disclaimer: The information provided in this article is for educational purposes only, and should not be considered medical advice. Please consult with a healthcare professional for more personalised guidance and various treatment options that may be available to you.
References:
- Center for Drug Evaluation and Research (no date) Medications containing semaglutide, U.S. Food and Drug Administration. Available at: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/medications-containing-semaglutide-marketed-type-2-diabetes-or-weight-loss (Accessed: 04 June 2023).
- Ries, J. (2023) Ozempic and Wegovy: FDA warns about rise of off-brand versions, Healthline. Available at: https://www.healthline.com/health-news/fda-issues-warning-about-off-brand-versions-of-ozempic-and-wegovy (Accessed: 04 June 2023).
- FDA warns people to avoid compounded semaglutide medicines (2023) WebMD. Available at: https://www.webmd.com/diabetes/news/20230531/fda-warns-people-avoid-compounded-semaglutide-medicines (Accessed: 04 June 2023).