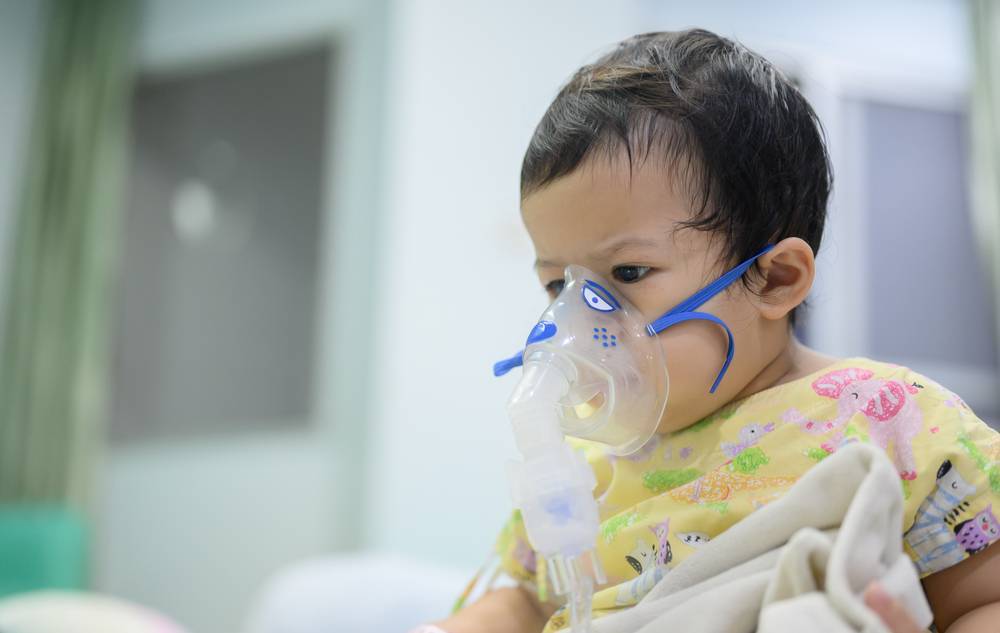
Sanofi and AstraZeneca have scored a landmark victory with the FDA approval of their novel respiratory syncytial virus (RSV) preventive treatment, Beyfortus.
This therapy marks a new milestone in infant and toddler healthcare, especially in preventing lower respiratory tract disease among this vulnerable population.
As partners in this medical breakthrough, Sanofi and AstraZeneca are set to transform RSV, or Respiratory Syncytial Virus, to preventive care. Beyfortus, their antibody therapy, has gained approval for shielding infants born during or entering their first RSV season and children up to 24 months of age who are still susceptible to severe RSV disease.
What is RSV?
RSV is a common respiratory virus that usually causes mild, cold-like symptoms. It’s most common in infants and young children, though it can affect people of all ages. In certain populations, such as premature infants, older adults, people with heart and lung disease, or those with weakened immune systems, RSV can lead to severe disease.
Symptoms of RSV typically appear in stages and not all at once. In very young infants with RSV, the only symptoms may be irritability, decreased activity, and breathing difficulties. In older infants and young children, typical symptoms may include:
- Runny nose
- Decreased appetite
- Coughing
- Sneezing
- Fever
- Wheezing
Symptoms differ for those suffering in severe cases:
- Severe cough
- High fever
- Cyanosis (bluish colour of skin, lips, and nails due to lack of oxygen)
- Rapid breathing or difficulty breathing
- Struggling to breathe or apnoea (pauses in breathing)
Certain individuals are at a high risk for severe RSV disease. These include:
- Infants and young children, particularly those under 1 year of age with certain risk factors (premature birth, congenital heart disease, chronic lung disease)
- Older adults, especially those with chronic heart or lung disease
- People with weakened immune systems
RSV is spread through respiratory droplets from a cough or sneeze. It can also be spread by direct contact with a contaminated surface.
If your child or someone you know is showing symptoms of severe RSV, such as difficulty breathing, bluish colour of the skin, or high fever, it’s important to seek medical attention immediately.
Making a Seasonal Impact with Beyfortus
Sanofi and AstraZeneca have strategically scheduled the launch of Beyfortus in the United States to precede the forthcoming RSV season. The pricing details for this preventive therapy will be released in sync with its market availability, ensuring informed access for its potential users.
RSV poses a considerable threat to infants, leading to a significant number of hospitalisations each year. Prior to Beyfortus, the primary preventive RSV therapy available was Swedish Orphan Biovitrum’s Synagis, designed specifically for high-risk infants.
Beyfortus: An Advancement Over Existing Therapies
The introduction of Beyfortus heralds a significant evolution in RSV therapy. Unlike Synagis, which requires monthly injections, Beyfortus offers extended coverage with a single dose per season. This long-acting therapy extends its preventive benefits to infants irrespective of their additional medical conditions.
FDA Approval Rooted in Convincing Evidence
Beyfortus, a laboratory-made protein known as a monoclonal antibody, has shown promising results in protecting against respiratory syncytial virus (RSV) infections. Administered as a single injection before or during RSV season, Beyfortus has been found to reduce the risk of severe RSV infections in infants and young children.
Three clinical trials (Trials 03, 04, and 05) supported the safety and effectiveness of Beyfortus. In Trial 03, Beyfortus reduced the incidence of medically attended RSV lower respiratory tract infections (MA RSV LRTI) by approximately 70% compared to a placebo. The trial included 1,453 preterm infants born during or entering their first RSV season.
Trial 04 focused on 1,490 term and late preterm infants and demonstrated a reduction of around 75% in the risk of MA RSV LRTI with Beyfortus compared to placebo. Trial 05, involving 925 preterm infants and infants with chronic lung disease or congenital heart disease, supported the use of Beyfortus for preventing MA RSV LRTI in this vulnerable population.
Although Beyfortus has shown positive results, it is essential to be aware of potential side effects such as rash and injection site reactions. It is contraindicated in infants and children with a history of serious hypersensitivity reactions to its ingredients. Precautions should also be taken for individuals with bleeding disorders.
Like all medical interventions, Beyfortus comes with certain caveats. The FDA has issued warnings about potential serious hypersensitivity reactions, urging caution in administering this therapy to infants and children with significant bleeding disorders.
A Positive Stride in Infant Healthcare
With Beyfortus’ FDA approval, Sanofi and AstraZeneca are paving the way for improved preventive care for RSV, a common and potentially severe disease in infants. This new therapy promises to significantly impact the field of paediatric healthcare, marking a crucial step forward in infant health and wellbeing.
References:
- FDA Approves New Drug to Prevent RSV in Babies and Toddlers. (2023, July 17). FDA Approves New Drug to Prevent RSV in Babies and Toddlers | FDA. https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-prevent-rsv-babies-and-toddlers
- PR: FDA approves BeyfortusTM (nirsevimab-alip) to protect infants against RSV disease. (n.d.). PR: FDA Approves BeyfortusTM (Nirsevimab-alip) to Protect Infants Against RSV Disease – Sanofi. https://1vynd53k72.execute-api.us-east-1.amazonaws.com/en/media-room/press-releases/2023/2023-07-17-17-00-00-2705911
- Beyfortus approved in the US for the prevention of RSV lower respiratory tract disease in infants (2023, July 17). https://www.astrazeneca.com/media-centre/press-releases/2023/beyfortus-approved-in-the-us-for-the-prevention-of-rsv-lower-respiratory-tract-disease-in-infants.html
- C. (2023, March 16). Learn about Respiratory Syncytial Virus Infection (RSV). Centers for Disease Control and Prevention. https://www.cdc.gov/rsv/index.html