CAR-T therapy, which reprograms a patient’s immune cells to attack cancer cells, has revolutionised the treatment of certain cancers. While successful in treating B-cell malignancies, its application to T-cell cancers, such as T-cell acute lymphoblastic leukaemia (T-ALL), posed unique challenges—until now.
At the forefront of overcoming these obstacles is CD7 CAR-T therapy, developed at the National University of Singapore’s Yong Loo Lin School of Medicine (NUS Medicine). Consequently, this innovation is providing new hope for patients who have exhausted all conventional treatment options.
The Rare and Aggressive Nature of T-ALL
T-ALL primarily affects children, adolescents, and young adults. It accounts for approximately 12-15% of childhood acute lymphoblastic leukaemia (ALL) and 25% of adult ALL cases.
In T-cell ALL, the bone marrow produces too many T-cells that are improperly formed and unable to effectively fight infections. These large numbers of T-cells prevent the patients from making the other blood cells their body needs.
The good news? The outlook for an individual with T-ALL is generally positive. “We cure about 85% of children and adults (with T-ALL)” shared Professor Allen Yeoh. He is the Head & Senior Consultant, Division of Paediatric Haematology and Oncology, Department of Paediatrics, Khoo Teck Puat – National University Children’s Medical Institute (KTP-NUCMI), National University Hospital (NUH).
The bad news – if they relapse, the chances of curing or surviving that relapse is very poor. The 5-year survival rate drops to about 7% in relapsed or refractory cases. Despite chemotherapy and bone marrow transplants, prognosis remains bleak .
“Majority (of patients) die within the first couple of months (after relapse),” explained Prof. Yeoh.
There is a pressing need to find new treatment options for this patient population.
Precision Cancer Treatment with CAR-T Therapy
Chimeric Antigen Receptor (CAR) T-cell therapy is a relatively new form of immunotherapy that harnesses the body’s immune system to fight cancer.
In this treatment, doctors first collect a patient’s T-cells and genetically engineer them to express a chimeric antigen receptor (CAR). After modification, they infuse the engineered T-cells back into the patient’s bloodstream, where the cells seek out and destroy cancer cells.
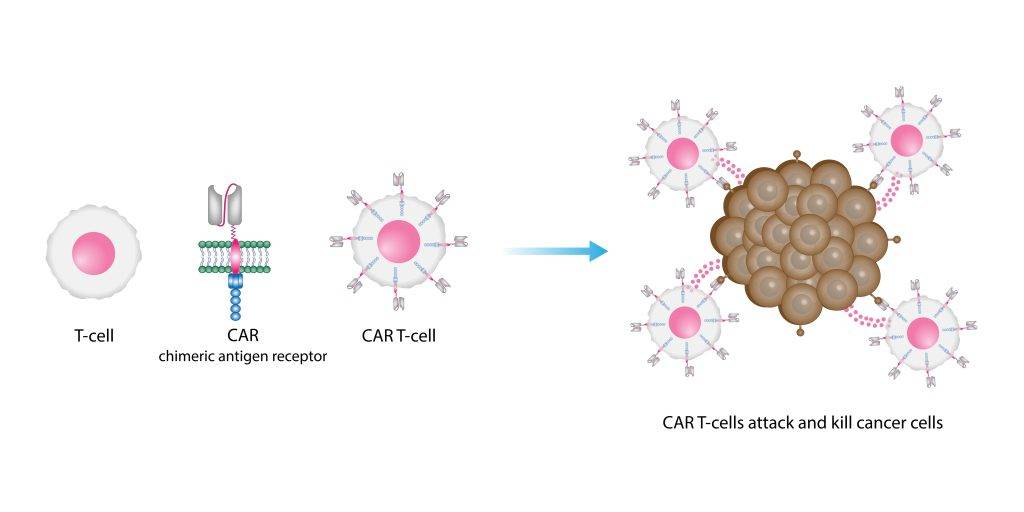
CAR-T therapy’s success depends on the CAR, which is designed to bind to a specific protein on the surface of cancer cells. Once bound, the CAR-T cells activate and trigger a strong immune response that directly targets the cancer cells, often resulting in rapid and significant reductions in disease activity.
CAR-T Therapy in CD19-Positive Cancers
For instance, one of the most significant advances in CAR-T therapy came with the development of CD19-targeting CAR-T treatments, which have shown remarkable efficacy in patients who had relapsed or were refractory to traditional treatments.
“The fascinating part about CAR-T cell therapy is that about 80% of these patients who are completely resistant to chemotherapy responded.” Prof. Yeoh shared.
“That means in a month or so, they respond, despite previous lack of response to chemotherapy and even bone marrow transplant.”
While CD19 CAR-T therapy has proven to be a game-changer in treating B-cell cancers, translating this success to T-cell cancers like T-ALL presented a unique set of challenges. As CAR-T therapy uses T-cells, these T-cells will also end up targeting itself and killing itself rather than the cancer cells.
The Innovative CD7 CAR-T Therapy
Developed by Professor Dario Campana and his team at NUS, CD7 CAR-T therapy employs an ingenious approach that aims to mitigate this problem.
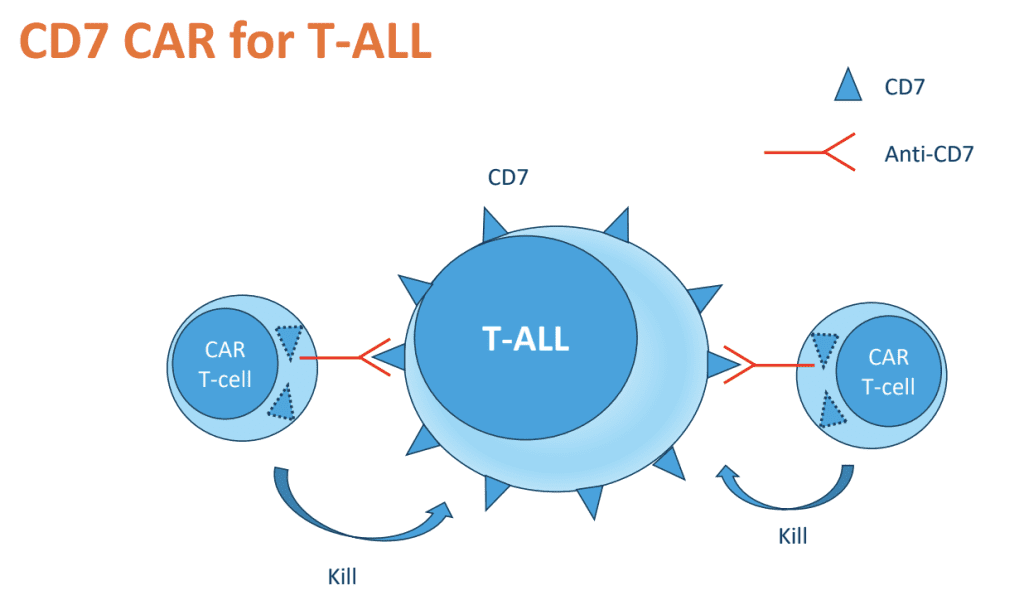
The innovative design hides the CD7 protein within the T-cells, preventing the CAR-T cells from recognising and attacking themselves. This technique allows the engineered T-cells to selectively target and destroy CD7-expressing leukaemic cells without harming themselves, hence “fratricide-resistant”.
Treatment Results and Patient Responses
The effectiveness of such CD7 CAR-T therapy has been remarkable, as published in Nature Medicine. The case series included 17 patients, ages ranging from 2 to 72, all of whom had T-ALL that had either relapsed or failed to respond to previous treatments.
16 of the 17 patients achieved remission within 1 month after CD7 CAR-T infusion.
CD7 CAR-T therapy has caused significantly milder side effects compared to traditional chemotherapy and existing CAR-T treatments targeting B-cell cancers.
“There is a significantly lower incidence of toxicity in the (T-ALL) CD-7 patients, despite having a much higher level of disease than in the CD-19 CAR patients. ” Dr. Bernice Oh shared, with reference to major side effects of cytokine release syndrome (CRS) and ICANS (a form of neurotoxicity)
She is the first author of the published study and a Consultant in the Division of Paediatric Haematology and Oncology at KTP-NUCMI, NUH.
Read also: The Inspiring Story of Three CD7 CAR-T Therapy Patients
The CD7 CAR-T Treatment Process
The process of CD7 CAR-T therapy is meticulous and involves several key steps to ensure the safety and efficacy of the treatment:
1. Harvesting (4 to 6 hours)
The treatment begins with the collection of a patient’s T-cells through a process called leukapheresis.
First, a dialysis-like machine connects to patients for 4 to 6 hours. During this process, the machine draws blood and separates the white blood cells, including T-cells, from the rest.
Afterward, the remaining blood is then infused back into the patient.
2. Quality Testing (2 to 3 weeks)
After harvesting, the T-cells undergo thorough quality testing to confirm they are suitable for the next steps in manufacturing. This process takes about 2 to 3 weeks.
3. Manufacturing (2 weeks)
The harvested T-cells are then genetically modified in the lab to express the CD7-targeting CAR. This step involves introducing a viral vector that carries the genetic material necessary for the CAR’s expression. The cells are multiplied in a controlled environment, generating a large quantity of CAR-T cells.
“The total manufacturing time is about two weeks for each CAR-T product that is specific to this specific patient.” Dr. Oh shared.
Dr. Oh leads the production of CD7 CAR-T cells in a fully-certified GMP facility, a small lab within NUS Medicine.
4. Quality Testing (2 to 3 weeks)
The CAR-T product undergoes another 2 to 3 weeks of quality testing before the clinical team decides that it is suitable for patient use.
5. Infusion (5 to 10 minutes)
After the two-week manufacturing process, the CAR-T cells are ready for infusion. At this point, the hospital admits patients about a week before the infusion and administers chemotherapy to boost the CAR-T cells’ effectiveness.
Next, the infusion process begins. The team administers the engineered T-cells through an IV line, which typically takes 5 to 10 minutes for a 15ml volume (about half a million CAR-T cells per kilogram).
Notably, only one infusion is needed.
6. Monitoring (2 to 3 weeks)
Following the infusion, patients are closely monitored for 2 to 3 weeks to check for adverse reactions and assess the therapy’s effectiveness.
Accelerating Innovation in Singapore
Singapore’s medical framework enables groundbreaking treatments like CD7 CAR-T therapy to reach patients faster than in other countries. As a result, this accelerated process gives patients who have exhausted conventional treatments timely access to experimental therapies.
Additionally, the Singapore Medical Council’s innovative salvage therapy guidance enables doctors to seek ethical approval for proposing new therapies that, though not yet widely adopted, show promising results.
“This CAR CD-7 (paper) was published in about 2017 by NUS. By 2019, we started the first patient.” Prof Yeoh shared.
In comparison, it took up to 15 years for the CAR-T cell therapy to be available for patients.

Prof Yeoh attributed the success of this rapid deployment is due to the collaborative efforts within Singapore’s academic health system, notably between NUS Medicine and the clinicians at NUHS.
“NUS being the academic university which discovered and patented this…and ourselves, the doctors in NUH being able to work together in this academic health system to really deliver this treatment within such a short timeframe.”
Academic and clinical teams, supported by Singapore’s innovative regulatory framework, have accelerated the delivery of CD7 CAR-T therapy to patients.
The Future of CD7 CAR-T Therapy
Building on these exciting results, the research team is actively recruiting for their clinical trial, CARTALL.
“We are fortunate that we have treated a number of patients using SMC ethical way, but it is not sufficient. We have to do it in a proper trial context.” Prof. Yeoh said. “We have to find which patient works, which patient does not work.”
Meanwhile, the research team is investigating the long-term durability of responses and side effects in patients who received the therapy. Prof. Yeoh remains confident about the research’s future direction.
“We have the technology, we have that capability…and we have the patient and the parents working with us.” He shared.
“We are only at the beginning of this exciting journey.”