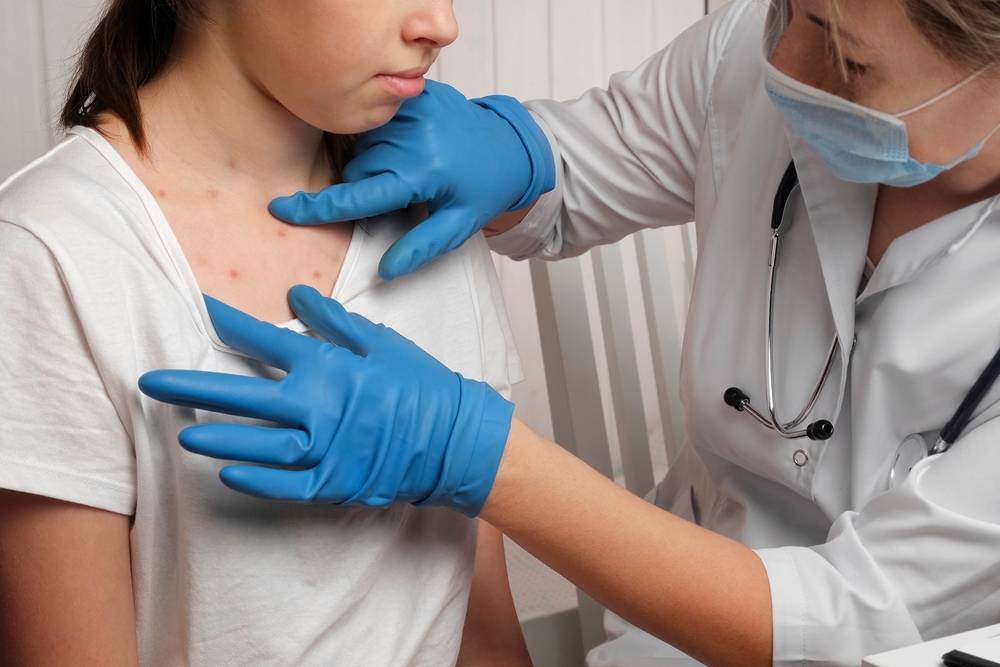
The new vaccine, trialled in Bangladesh, offers hope against the debilitating dengue virus.
The fight against dengue, a persistent health challenge in Asia, is seeing a promising light. A dengue vaccine, trialled in Bangladesh, demonstrates potential in combating the viral disease.
Pioneering Research in Bangladesh
Researchers from the icddr,b and the Larner College of Medicine at the University of Vermont (UVM), USA, have broken new ground by studying this dengue vaccine in Bangladesh. Their study on the single-dose tetravalent dengue vaccine candidate, TV005, showcased its safety and immune efficacy in children and adults. The findings of this trial have been featured in the esteemed journal, The Lancet Infectious Diseases.
A Glimpse into the Study
Under the “Dengue in Dhaka Initiative (DIDI)”, initiated in 2015, investigators from icddr,b and UVM’s Vaccine Testing Centre (VTC) set the ball rolling. 2015 also saw the establishment of clinical trials, laboratory assay infrastructure, and a preliminary study on dengue prevalence at the icddr,b. The trial roped in nearly 200 participants, aged between 1 and 49, over a span of three years from 2016. Researchers administered either the TV005 vaccine or a placebo to these volunteers.
The study revealed promising results: “TV005 was well-received. Most volunteers, post-vaccination, showed antibodies to all four dengue serotypes. Those with prior dengue infections exhibited higher antibody counts,” the study notes.
Such findings edge the TV005 vaccine closer to regular use in endemic areas, fuelling momentum for the ongoing, larger phase III efficacy trials.
A Long-standing Research Endeavour
The dedication to dengue research has been around for UVM VTC. They evaluated dengue vaccines crafted by the US National Institutes of Health (NIH) since 2009.
“Dengue vaccine development, especially an effective tetravalent one, is crucial for Bangladesh’s vast populace, currently grappling with severe dengue outbreaks,” remarked Rashidul Haque, a seasoned scientist at icddr,b. He remains optimistic that their efforts will fast-track the creation of dengue vaccines tailored for Bangladesh.
On the Global Front
Phase III trials for this dengue vaccine are already underway in India and several other nations. Mohammed Shafiul Alam, a scientist involved in the study, cautiously estimated that if all progresses well, the vaccine might be market-ready in the next three to five years.
With the vaccine having undergone trials in 42 distinct phases across various countries, Alam expressed optimism. “This presents a brilliant prospect for Bangladesh. Undoubtedly, it’s heartening news for the nation,” he concluded.
Current Treatment Limitations
Currently, managing symptoms and fluids is the sole treatment avenue for dengue patients. With this potential vaccine breakthrough, countless individuals may benefit from proactive disease prevention.
In Singapore, the United States and other countries, Dengaxia is the only vaccine against dengue. However, as stated on the Ministry of Health Singapore website, “the vaccine is not recommended for individuals who have no prior infection, because of the increased risk of developing severe dengue, should they get infected with dengue later in life. Due to Singapore’s relatively low dengue prevalence, most people are ineligible to receive the vaccine. As such, Dengvaxia vaccination is not an effective way to control dengue at the population level and Singapore does not track the number of people who have taken the vaccine.”