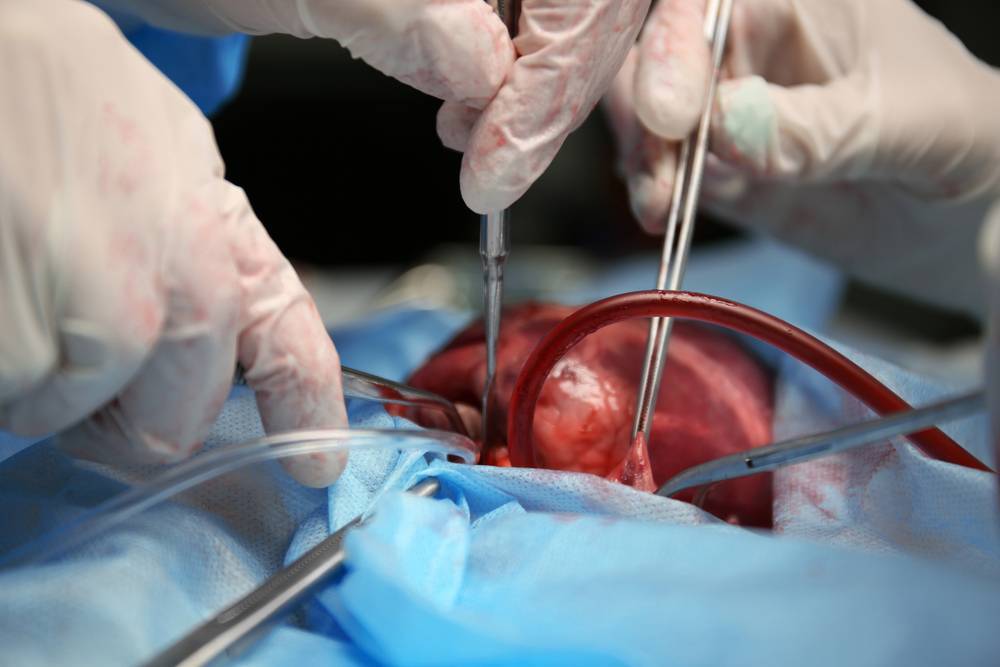
A month following the experimental pig heart transplant at the University of Maryland, the heart of a genetically modified pig continues to function optimally in its human host, showing no signs of rejection.
This achievement holds immense promise for addressing the shortage of human heart donors. It opens up the possibility of using pig hearts for transplantation, revolutionising the field of xenotransplantation.
Patient Spotlight: Lawrence Faucette
Lawrence Faucette, a 58-year-old individual who underwent the pioneering pig heart transplant in September, stands at the forefront of this medical breakthrough.
Ineligible for a traditional human heart transplant due to pre-existing conditions and heart disease, Faucette’s remarkable recovery offers hope for future patients in similar situations.
Pioneering Doctors: Dr. Bartley Griffith and Dr. Muhammad Mohiuddin
The medical team, led by Dr. Bartley Griffith and Dr. Muhammad Mohiuddin, has been closely monitoring Faucette’s progress. The heart’s ability to function independently, with the withdrawal of initial support drugs, marks a significant milestone. Efforts are now focused on enhancing Faucette’s physical strength with support from the physical therapy team.
Faucette’s Pig Heart Transplant Journey
Faucette’s journey from admission to the University of Maryland Medical Center on September 14, following heart failure symptoms, has been challenging yet inspiring.
Facing two cardiac arrests, he relied on an automatic defibrillator in his room to stay alive. The xenotransplant procedure emerged as his only hope, driven by a desire for more cherished moments with his family.
The Role of Genetic Modification and FDA Approval
The pig heart used in this procedure came from a genetically modified pig created by Revivicor, a subsidiary of the United Therapeutics Corporation. Genetic modifications included the inactivation of three genes to eliminate the alpha gal sugar in pig blood cells. This is a known trigger for severe human immune reactions.
Additionally, six human genes were added to the pig’s genome to enhance immune system acceptance. The FDA sanctioned this experimental pig heart transplant under its “compassionate use” program. This program offers hope to patients with severe or life-threatening conditions in the absence of alternative therapies.
Mitigating Immune Responses and Ongoing Monitoring
Faucette received experimental antibody treatment to further suppress immune system reactions and prevent rejection. Continuous monitoring for signs of rejection or pig-related viruses remains an essential part of his post-operative care.
This pig heart transplant not only highlights the potential of xenotransplants, but also instils hope among the more than 113,000 individuals on the organ transplant waiting list in the US. While the journey is still in its early stages, it holds the promise of revolutionising cardiac care and bringing the dream of reducing the heart transplant waiting list closer to reality.
Reference
- Neergaard, L. (2023, October 20). A month after a pig heart transplant, man works to regain strength with no rejection so far | AP News. AP News. https://apnews.com/article/pig-heart-transplant-maryland-a25576010ff2b7915b53e8144c3dd3cf