
Weekly Asian Medical News Bulletin – 2 Dec 2022
This week we look at HIV on World Aids Day the world s eight billion



This week we look at HIV on World Aids Day the world s eight billion
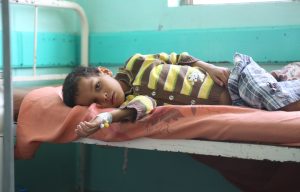
Cholera a potentially fatal waterborne disease continues to pose a significant health risk in many

Discover the link between diet and mental health A healthy diet that is rich in

Experts sound the alarm as over six million skilled professionals, including vital healthcare workers, have left Pakistan since 1971, severely impacting the nation’s development and future prospects.

Heavy rains and consecutive flooding events triggered by typhoons and the southwest monsoon (“habagat”) are linked to a sharp rise in leptospirosis cases in the Philippines. In just one month, there’s been a 139% increase nationwide, according to the Department of Health (DOH).

Malaria, a mosquito-borne infectious disease, has plagued humanity for centuries. Despite advances in medical science, it remains a persistent public health challenge, particularly in Asia and the Middle East. In this article, we explore the dynamics of malaria, its prevalence in the region, and the ongoing efforts to eradicate this deadly disease.




