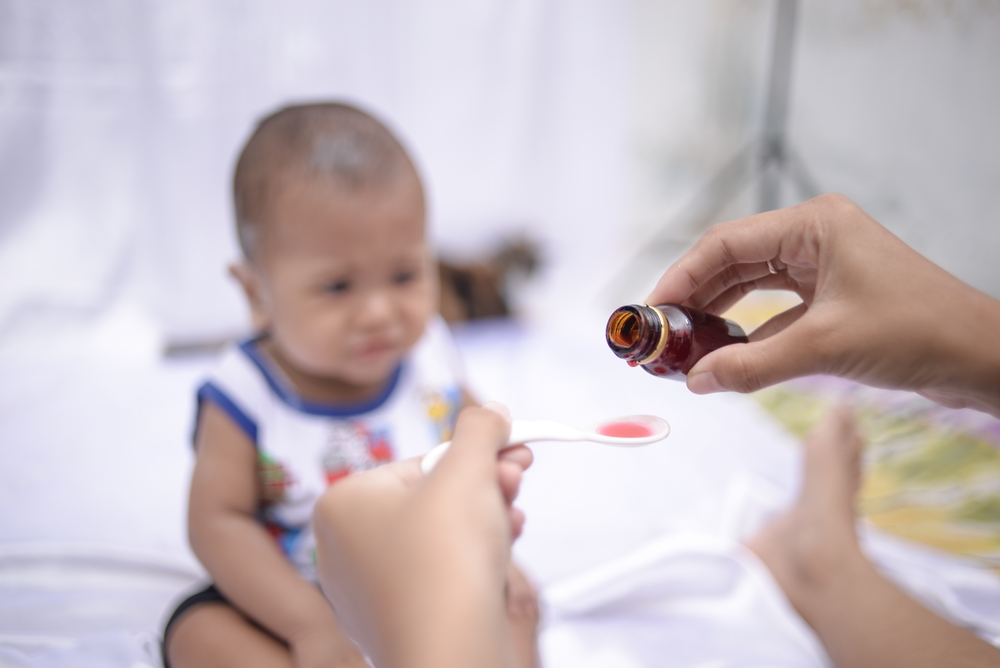
In a shocking revelation, Indonesian pharmaceutical company Afi Farma stands accused of using almost pure toxin in 70 batches of cough syrup.
This led to the tragic deaths of more than 200 children last year, as detailed in court documents.
The company, based in Kediri, East Java, allegedly used ingredients with toxin concentrations up to 99%, prompting an urgent review of global drug supply chain oversight.
Global Concerns Over Drug Safety
This alarming situation isn’t isolated to Indonesia. Similar poisoning cases due to contaminated cough syrups have caused numerous child fatalities globally, notably in Gambia and Uzbekistan. These incidents have accelerated international efforts to fortify drug supply chain regulations.
Toxic Ingredients in Cough Syrup
Investigations reveal that between October 2021 and February 2022, Afi Farma purportedly produced cough syrup using propylene glycol that contained an astounding 96% to 99% ethylene glycol (EG), a harmful substance. The prosecutor, Ikhsan Nasrulloh, confirmed to Reuters that these findings resulted from police testing conducted last year.
Defence and Regulatory Loopholes
Afi Farma’s legal representative, Reza Wendra Prayogo, defended the company, stating there is no evidence of deliberate poisoning. He highlighted that Indonesia’s drug authority, BPOM, doesn’t enforce stringent testing of pharmaceutical ingredients. Remarkably, a 2018 BPOM guideline permitted drug producers to depend on suppliers’ tests, conducting only minimal “identification tests” without mandatory toxicity checks. BPOM has yet to comment on the issue.
Upcoming Court Proceedings
The case against Afi Farma, among others implicated in distributing toxic cough syrups, advances to court on October 18. The World Health Organization (WHO) states that global standards dictate the safe limit for the known toxins EG and diethylene glycol (DEG) to be no more than 0.10%. Similarly, Indonesia’s health ministry adopted these limits in its 2020 drug standards guidelines.
The Dangers of EG and DEG
EG, used in products like antifreeze and car de-icing solutions, poses severe health risks, potentially triggering acute kidney injury if ingested. Experts explained to Reuters that cost-saving malpractices could lead producers to substitute propylene glycol with cheaper, hazardous chemicals EG and DEG.
Afi Farma Faces Consequences
Post-incident, authorities revoked Afi Farma’s drug manufacturing licence and removed its products from the market for non-compliance with manufacturing regulations. Further, the company’s top officials, including the CEO and quality control manager, face negligence charges. They allegedly failed to perform necessary safety checks, relying instead on supplier-provided safety certificates. Prosecutors are pursuing up to nine years of imprisonment for these individuals.
Despite these accusations, Afi Farma’s legal team denies any wrongdoing. The broader repercussions of this scandal have ignited criminal investigations, lawsuits, and heightened regulatory scrutiny around the globe, particularly following reports of similar negligence among some Indian pharmaceutical firms.