
Ovarian Cancer Day: Top 5 Celebrities Who Battled the Silent Killer
World Ovarian Cancer Day is a global initiative to raise awareness about ovarian cancer, a



World Ovarian Cancer Day is a global initiative to raise awareness about ovarian cancer, a
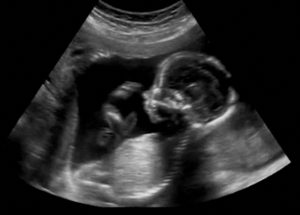
In a medical breakthrough, doctors in Boston have successfully performed pioneering in-utero brain surgery, treating

Get the best help for Osteoarthritis with the guidance of physiotherapists – #WorldPTDay World Physiotherapy

World First Aid Day (WFAD), conducts various activities held to raise awareness of the life-saving role of first aid.

Nutritional deficiencies occur when the body lacks certain micronutrients i.e. vitamins and/or minerals. Micronutrients are vital to life in many ways e.g.: Optimal growth and

As our feet carry our bodies’ weights and are more prone to injuries it is important to know the various conditions that could occur.




