

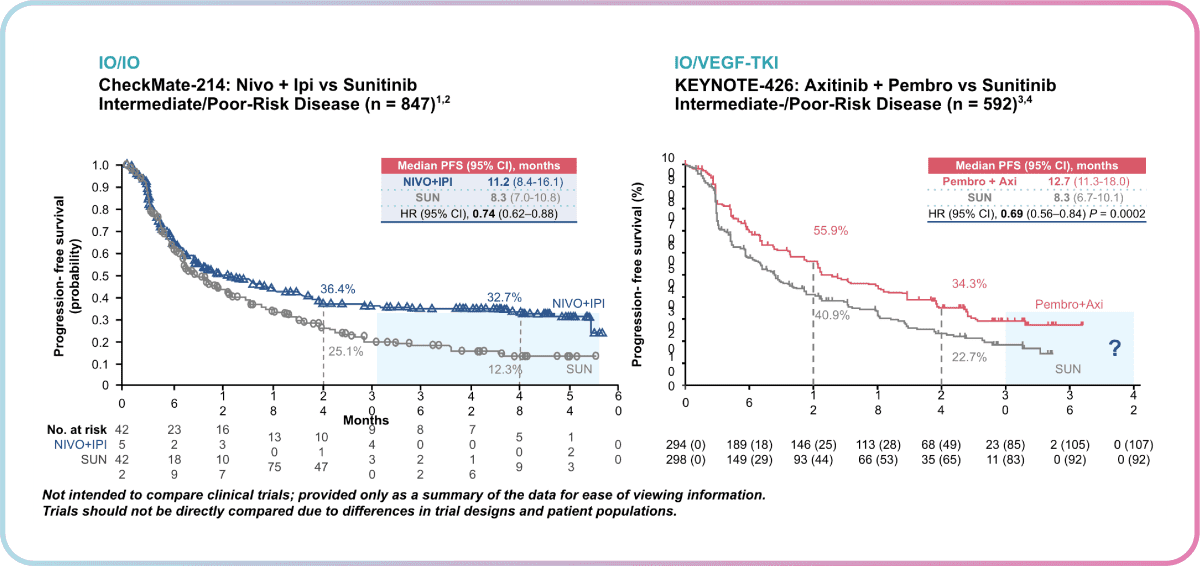
Dr Heng highlighted the importance of this observation in showing a durable response to treatment with Ipilimumab + Nivolumab, where patients are unlikely to progress further. However, KEYNOTE-426 patients have only been followed for 24 months in the most recent analysis, thus close monitoring over a longer period will be needed to shed more insight into the possibility of the “tail of the curve” observation for Pembrolizumab + Axitinib as well.
Additionally, a notable difference was observed between the shape of curves at the beginning of therapy. Unlike Ipilimumab + Nivolumab, a good upfront response was observed for Pembrolizumab + Axitinib. This translates to clinical setting decisions, where a trade-off between a good upfront response and a durable response exists.
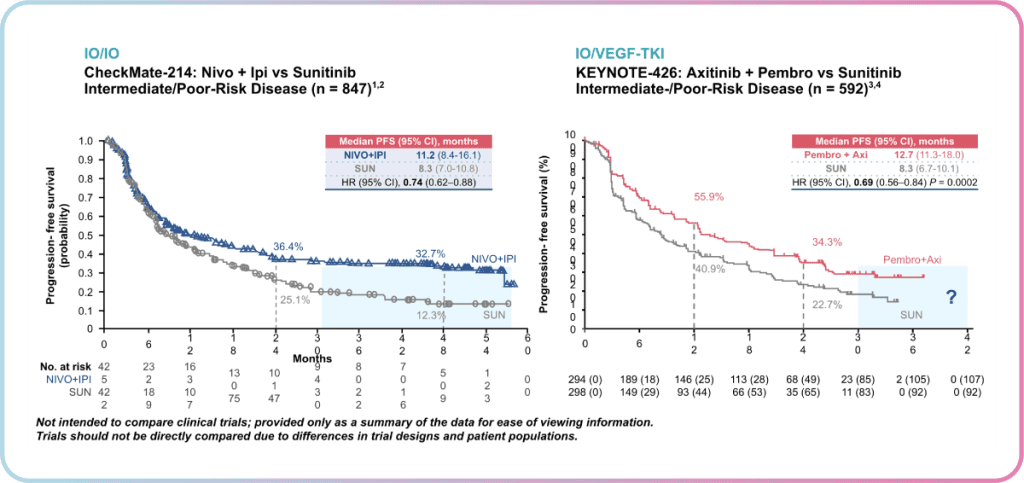
Dr Heng highlighted the importance of this observation in showing a durable response to treatment with Ipilimumab + Nivolumab, where patients are unlikely to progress further. However, KEYNOTE-426 patients have only been followed for 24 months in the most recent analysis, thus close monitoring over a longer period will be needed to shed more insight into the possibility of the “tail of the curve” observation for Pembrolizumab + Axitinib as well.
Additionally, a notable difference was observed between the shape of curves at the beginning of therapy. Unlike Ipilimumab + Nivolumab, a good upfront response was observed for Pembrolizumab + Axitinib. This translates to clinical setting decisions, where a trade-off between a good upfront response and a durable response exists.
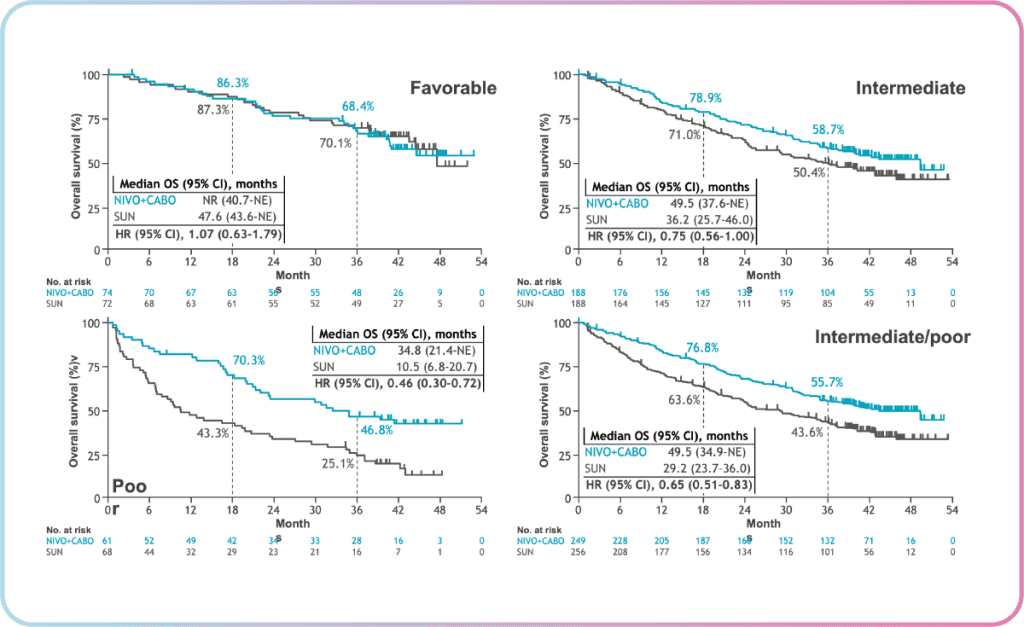
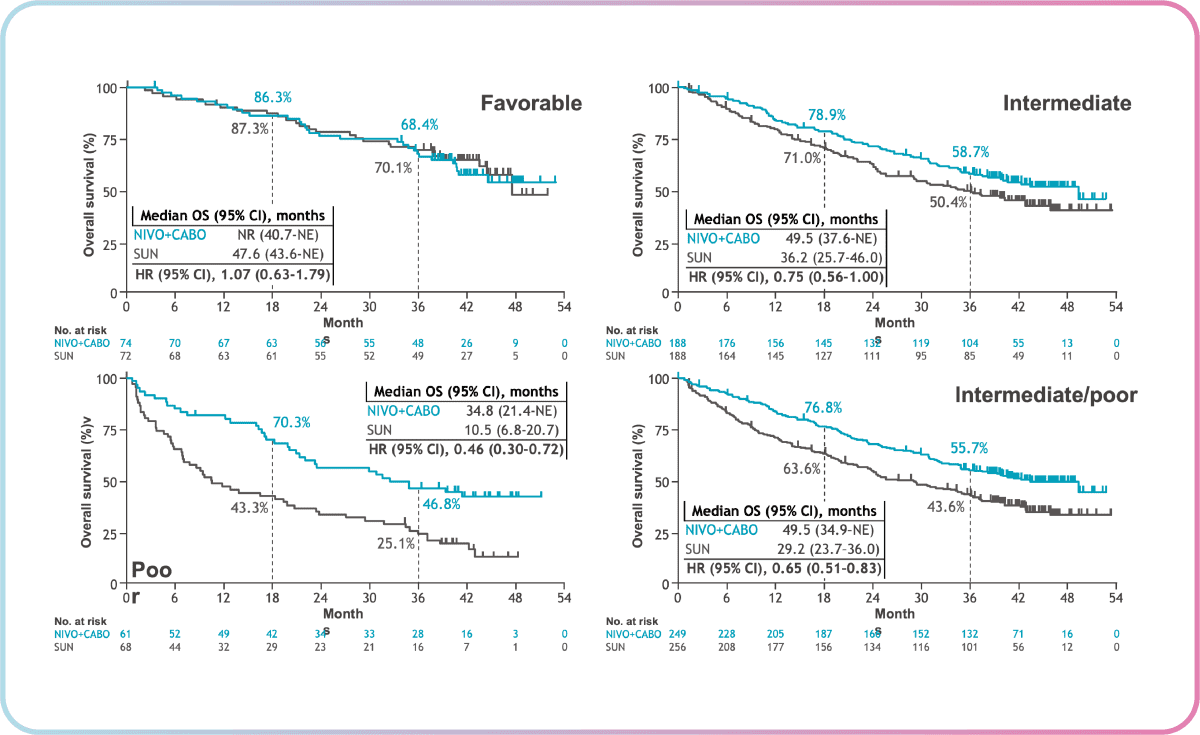
In Checkmate 9ER, there is an inkling of a “tail of the curve” observation in the overall survival for Nivolumab + Cabozantinib, which is similar to that of Ipilimumab + Nivolumab. Dr Heng notes that it is still too early to tell but the potential is there as Nivolumab + Cabozantinib has been efficacious thus far.
In Checkmate 9ER, there is an inkling of a “tail of the curve” observation in the overall survival for Nivolumab + Cabozantinib, which is similar to that of Ipilimumab + Nivolumab. Dr Heng notes that it is still too early to tell but the potential is there as Nivolumab + Cabozantinib has been efficacious thus far.
From the Checkmate 9ER 2023 data shared by Dr Heng, the combination of Nivolumab + Cabozantinib showed overall survival benefit in IMDC intermediate/poor and poor-risk patients (as shown by the lower hazard ratios in figure 2). These results continue to support nivolumab + cabozantinib as a first-line treatment for patients with advanced RCC.
To answer an interesting question raised by the floor: “Is it a sin to use VEGF monotherapy instead of combination therapy?”, Dr Heng shared from his clinical experience that he prefers to start patients who are in the favourable risk group (IMDC score 0) on combination VEGF/IO therapy, especially the newer combinations, to achieve better efficacy. While it is possible for such patients to do well on VEGF monotherapy long-term, it remains unpredictable as to when their condition may deteriorate. Instead, VEGF monotherapy such as Pazopanib can be reserved for frail or elderly patients due to a more well-tolerated side effect profile.
While the impact on survival rates is important, Dr Heng stated that many patients ranked QoL as one of the most important factors to consider when choosing a therapy. Compared to Sunitinib monotherapy, Nivolumab + Cabozantinib showed consistent benefits across global standardised health-related quality-of-life measures in different scales such as EQ-5D, FKSI-19 and FKSI-DRS. The time to deterioration in the functional assessment of cancer therapy-kidney symptom index (FKSI-19), a QoL marker specific to kidney disease, was also improved. This was not observed for other combinations such as Lenvatinib + Pembrolizumab and Pembrolizumab + Axitinib.
As new clinical data is collected and real-world evidence emerges, Dr Heng concludes his presentation by sharing his excitement about the potential updates we can look out for in the treatment of mRCC.
From the Checkmate 9ER 2023 data shared by Dr Heng, the combination of Nivolumab + Cabozantinib showed overall survival benefit in IMDC intermediate/poor and poor-risk patients (as shown by the lower hazard ratios in figure 2). These results continue to support nivolumab + cabozantinib as a first-line treatment for patients with advanced RCC.
To answer an interesting question raised by the floor: “Is it a sin to use VEGF monotherapy instead of combination therapy?”, Dr Heng shared from his clinical experience that he prefers to start patients who are in the favourable risk group (IMDC score 0) on combination VEGF/IO therapy, especially the newer combinations, to achieve better efficacy. While it is possible for such patients to do well on VEGF monotherapy long-term, it remains unpredictable as to when their condition may deteriorate. Instead, VEGF monotherapy such as Pazopanib can be reserved for frail or elderly patients due to a more well-tolerated side effect profile.
While the impact on survival rates is important, Dr Heng stated that many patients ranked QoL as one of the most important factors to consider when choosing a therapy. Compared to Sunitinib monotherapy, Nivolumab + Cabozantinib showed consistent benefits across global standardised health-related quality-of-life measures in different scales such as EQ-5D, FKSI-19 and FKSI-DRS. The time to deterioration in the functional assessment of cancer therapy-kidney symptom index (FKSI-19), a QoL marker specific to kidney disease, was also improved. This was not observed for other combinations such as Lenvatinib + Pembrolizumab and Pembrolizumab + Axitinib. As new clinical data is collected and real-world evidence emerges, Dr Heng concludes his presentation by sharing his excitement about the potential updates we can look out for in the treatment of mRCC.
References 1. Tannir. ASCO GU 2020. Abstr 609; 2. Albiges, et al. ESMO Open. 2020;5(6):e001079; 3. Plimack. ASCO 2020. Abstr 5001; 4. Powles T, et al. Lancet Oncology. 2020; 21(12):1563 1573. 5. Porta C, et al. Poster 668P. ESMO 2021; 6. Cella D, et al. Lancet Oncol 2022;doi:10.1016/S1470-2045(21)00693-8; 7. Burotto M et al GU ASCO 2023.
CMX-SG-000493
References 1. Tannir. ASCO GU 2020. Abstr 609; 2. Albiges, et al. ESMO Open. 2020;5(6):e001079; 3. Plimack. ASCO 2020. Abstr 5001; 4. Powles T, et al. Lancet Oncology. 2020; 21(12):1563 1573. 5. Porta C, et al. Poster 668P. ESMO 2021; 6. Cella D, et al. Lancet Oncol 2022;doi:10.1016/S1470-2045(21)00693-8; 7. Burotto M et al GU ASCO 2023.
CMX-SG-000493




