
Unmasking Endocrine Disruptors: The Invisible Chemicals Shaping Our Health
Endocrine-disrupting chemicals (EDCs) are a silent menace that infiltrate our daily lives, impacting our health

Endocrine-disrupting chemicals (EDCs) are a silent menace that infiltrate our daily lives, impacting our health
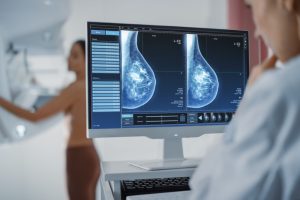
Recently, a group of Swedish researchers have shown that AI-supported mammography screening resulted in a
Blood donation is the voluntary act of giving blood, mainly for transfusion. Blood transfusion replaces

Gao dedicated herself to helping AIDS patients, often at great personal cost. She distributed educational leaflets, provided medical assistance, and vocally criticised the local government for covering up the crisis. This stance eventually led to conflicts with officials and her subsequent move to the United States.

With telemedicine on the rise, various employers and government agencies have raised concerns regarding the excessive issuing of medical certificates (MCs). How does this discussion of MCs correlate with the well-being of employees in the workplace?
Breast cancer is the uncontrolled growth of abnormal cells that develop in the breasts. Signs may include noticing a lump in one or both breasts,




